Country seals deal for 5m doses of Chinese-developed shot. World Health Organisation has given an emergency use listing to Sinopharm Chinas Covid-19 vaccine.

Covid 19 Different Types Of Vaccines And How They Work European Science Media Hub
Currently the EU has approved four vaccines Pfizer-BioNTech Moderna Oxford-AstraZeneca and JanssenJohnson Johnson.

Vaccine sinopharm europe. The safety updates summarise the data that have become available since the vaccines authorisation. The sinopharm vaccine which has been readily used in the UAE Bahrain and other Middle East countries is awaiting official approval from the WHO. Both use inactivated viruses to prompt an immune response in.
The Sinopharm vaccine contains SARS-CoV-2 that has undergone treatment with. Europe News -A study found the Sinopharm vaccine has an efficacy of 84 per cent after the application of its two doses. This country is among 212 other countries using Sinopharm and AstraZeneca vaccines which the European Union isnt allowing for travel entry but the situation is fluid ever changing and is the subject of policy discussions around the world.
The World Health Organization WHO approved a COVID-19 vaccine from Chinas state-owned drugmaker Sinopharm for emergency use on Friday a boost to Beijings push for a. Beijing Institute of Biological Products Co Ltd. Also consultations on the future of the National Insurance NIS system will first involve employers.
China has two vaccines authorized for emergency use by the World Health Organization WHO Sinopharm and Sinovac. In EU first Sinopharms coronavirus vaccine approved by Hungary. The Council of Ministers decided on Thursday to include Chinese Sinopharm in the list of acceptable vaccines for entry in the countryMinister of Transport Yiannis Karousos said after the Cabinet meeting that all persons regardless of nationality who have been vaccinated and hold a vaccination certificate from the EU Member States - including the Republic of Cyprus the countries.
Vaccination gathers pace across the European Union. BIBP COVID-19 Vaccine by China National Biotec Group CNBG Sinopharm International non. Cyprus on Thursday added Chinese Covid vaccine Sinopharm to the list of approved vaccines accepted for entry into the country.
Sinopharm COVID-19 vaccines International non-proprietary name. Deliveries of vaccine doses to European Union countries have increased steadily since December. The WHO Strategic Advisory Group of Experts SAGE on Immunization has issued Interim recommendations for the use of the inactivated COVID-19 vaccine BIBP developed by SinopharmChina National Pharmaceutical Group.
The study published in the Journal of the American Medical Association on Wednesday has finally answered. The WHO has approved AstraZeneca Pfizer Janssen Moderna Sinopharm and Sinovac vaccines for emergency use. The two-dose vaccine incorporates inactivated virus to stimulate an immune response.
EMA releases a monthly update for each authorised COVID-19 vaccine. Here is what you need to know. The Chinese drug maker Sinopharm has finally published the interim results from a phase 3 trial of its Covid-19 vaccine after more than 200 million doses have been administered worldwide.
BIBP-CorV Fact sheet for health workers. Hungary became the European Unions first member to approve Chinas. It said all the vaccines it had approved for.
The Commission has so far given 4 conditional marketing authorisations for the vaccines developed by BioNTech and Pfizer Moderna AstraZeneca and Janssen Pharmaceutica NV following EMA positive assessment of their safety and efficacy. EMA publishes safety updates for the COVID-19 vaccines authorised in the EU. The World Health Organisation has given an emergency use listing to Sinopharm Chinas Covid-19 vaccine making it the sixth vaccine to receive validation.
Meanwhile on 7 May the World Health Organization WHO listed a Sinopharm Covid-19 vaccine for emergency use for adults 18 years and older. WHO approves Sinopharm vaccine boosting Gulf-European travel hopes. BUDAPEST REUTERS - Sinopharms Covid-19 vaccine was less effective in offering protection against the disease among the elderly according to the results of a Hungarian study.
They also indicate whether any safety information requires further investigation. Sinopharm is a state-owned Chinese pharmaceutical company and is trialling two Covid-19 vaccines in countries including UAe Argentina and Morocco.

Sinopharm S Covid 19 Vaccine Receives 1st Eu Gmp Certificate From Hungary To Be More Competitive In Intl Market Global Times

Five Hurdles In The Global Covid 19 Vaccine Rollout European Data News Hub

Vaccines Turn Into Geopolitics In Europe S Most Volatile Region Bloomberg
11 Ivermectin Drug Is Not Effective At Treating Mild Covid 19 Study Finds
Sinopharm S Covid 19 Vaccine Receives 1st Eu Gmp Certificate From Hungary To Be More Competitive In Intl Market Global Times

Serbia Outperforms Eu W Balkans In Covid Vaccination

Eu Exported 34m Vaccines Since January Despite Internal Shortfalls Cgtn

Hungary Reaches Deal To Buy China S Sinopharm Vaccine Euractiv Com

After Sinopharm Eu Does Not Recognize Indian Version Of Astrazeneca Vaccine

Hungary Becomes First Eu Country To Receive China S Sinopharm Vaccine Cgtn

Which Eu Countries Accept Vaccinated Travelers Europe News And Current Affairs From Around The Continent Dw 08 06 2021

Which Eu Countries Accept Vaccinated Travelers Europe News And Current Affairs From Around The Continent Dw 08 06 2021

Sinopharm Coronavirus Vaccine Who Grants Emergency Use Authorization For Chinese Made Vaccine The Washington Post

Hungary Becomes First Eu Country To Receive China S Sinopharm Vaccine Cgtn
Hungary To Produce China S Sinopharm Covid 19 Vaccine By 2022 Cgtn
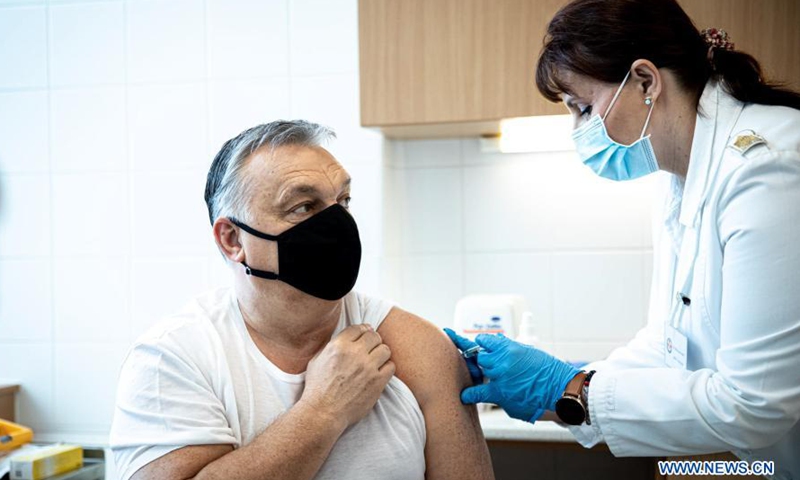
Sinopharm S Covid 19 Vaccine Receives 1st Eu Gmp Certificate From Hungary To Be More Competitive In Intl Market Global Times
Eu Likely To Decide On Moderna Covid Shot For Kids Next Week
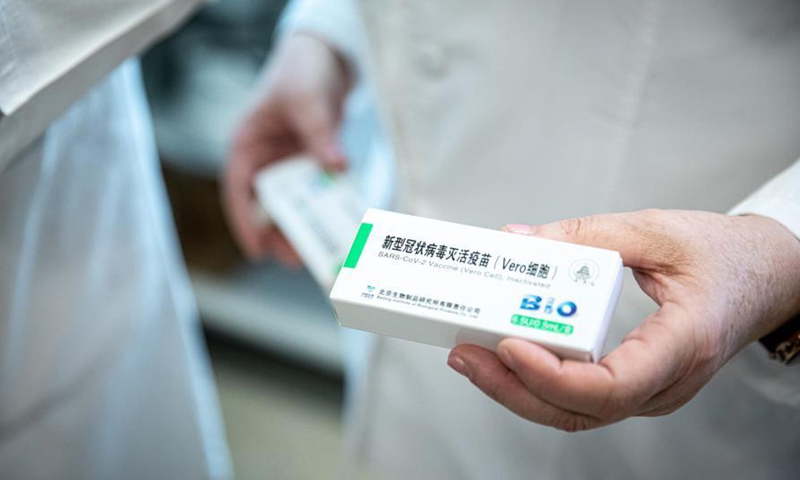
Sinopharm S Covid 19 Vaccine Receives 1st Eu Gmp Certificate From Hungary To Be More Competitive In Intl Market Global Times